
Electrochemical Interfaces at the Atomic Scale
Electrochemical interfaces are the hidden stage where the most important energy-conversion reactions unfold.
They dictate the efficiency, durability, and selectivity of devices ranging from fuel cells and electrolyzers
to advanced batteries. Understanding and controlling these buried boundaries between electrodes, solvents,
and ions is essential for building the next generation of sustainable energy technologies.
At the Harlow Group, we approach this challenge by bridging fundamental electrochemistry,
surface science, materials design, and physics. We combine single-crystal electrochemistry
with in situ synchrotron x-ray scattering, laser-induced temperature jumps,
and ultra-high-vacuum techniques to capture how interfaces evolve under reaction conditions.
By uniting precision experiments with modern analysis tools, our goal is to reveal atomic-scale mechanisms
and provide guiding principles for engineering new electrochemical materials.

Gary (PI), Yiduo*, Aden (undergrad), Tyler (undergrad). *OCE M.S. students
Research Areas

Advanced X-ray & Neutron Methods
Electrochemical interfaces restructure with potential and electrolyte composition. We specialize in
in situ surface x-ray diffraction (SXRD) for sub-ångström surface structures under operating
conditions, complemented by grazing-incidence XAFS, coherent Bragg diffraction, and in situ XPS.
Prof. Harlow developed the HAT software package
for high-energy SXRD data treatment.
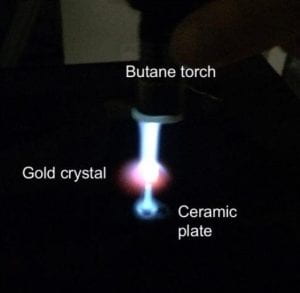
Single-Crystal Electrochemistry
Atomically flat facets let us link surface structure to kinetics, overpotentials, and
product selectivity. We employ rigorously cleaned glassware/cells and precise electrode preparation
(flame annealing, UHV sputter/anneal, induction heating). A new UHV → electrochemistry transfer
system is underway for ultimate surface control.
Molecularly Modified Electrodes
Covalently bound organic layers tune electronic structure, solvation, and
reaction pathways. We combine single-crystal studies with LITJ, in situ SXRD,
and spectroscopy to design metal–molecule interfaces for targeted function.
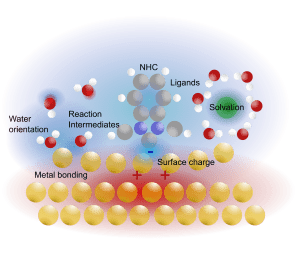
Liquid Structure at Interfaces
Specular and non-specular scattering yield electron density profiles across the double layer.
Using contrast variation (electrolyte concentration and strongly scattering cations), we access fundamental insights
into ion and solvent organization at electrified interfaces.

Model Oxides & Alloys for Electrocatalysis
With international collaborators, we prepare well-defined oxides/alloys via MBE and characterize
atomic structures in situ at synchrotrons using high-energy SXRD, informing mechanisms relevant
to water splitting and related reactions.

Hierarchical Nanostructures
We use templated electrodeposition to build architectures otherwise inaccessible, creating catalyst
layers for CO₂ reduction, electrolysis, and beyond.
Funding
Our work is supported by the U.S. Department of Energy · Basic Energy Sciences.
